In 2004, Gilead Sciences decided to stop pursuing a new HIV drug. The public explanation was that it wasn’t sufficiently different from an existing treatment to warrant further development.
In private, though, something else was at play. Gilead had devised a plan to delay the new drug’s release to maximise profits, even though executives had reason to believe it might turn out to be safer for patients, according to a trove of internal documents made public in litigation against the company.
Gilead, one of the world’s largest drugmakers, appeared to be embracing a well-worn industry tactic: gaming the US patent system to protect lucrative monopolies on best-selling drugs.
At the time, Gilead already had a pair of blockbuster HIV treatments, both of which were underpinned by a version of a drug called tenofovir. The first of those treatments was set to lose patent protection in 2017, at which point competitors would be free to introduce cheaper alternatives.
The promising drug, then in the early stages of testing, was an updated version of tenofovir. Gilead executives knew it had the potential to be less toxic to patients’ kidneys and bones than the earlier iteration, according to internal memos unearthed by lawyers who are suing Gilead on behalf of patients.
Despite those possible benefits, executives concluded that the new version risked competing with the company’s existing, patent-protected formulation. If they delayed the new product’s release until shortly before the existing patents expired, the company could increase the period of time in which at least one of its HIV treatments remained protected by patents.
The “patent extension strategy”, as the Gilead documents repeatedly called it, would allow the company to keep prices high for its tenofovir-based drugs. Gilead could switch patients to its new drug just before cheap generics hit the market. By putting tenofovir on a path to remain a money-making juggernaut for decades, the strategy was potentially worth billions.
Gilead ended up introducing a version of the new treatment in 2015, nearly a decade after it might have become available if the company had not paused development in 2004. Its patents now extend until at least 2031.
The delayed release of the new treatment is now the subject of lawsuits in which some 26,000 patients who took Gilead’s older HIV drugs claim that the company unnecessarily exposed them to kidney and bone problems.
In court filings, Gilead’s lawyers said that the allegations were meritless. They denied that the company halted the drug’s development to increase profits. They cited a 2004 internal memo that estimated Gilead could increase its revenue by $1 billion over six years if it released the new version in 2008.
“Had Gilead been motivated by profit alone, as plaintiffs contend, the logical decision would have been to expedite” the new version’s development, the lawyers wrote.
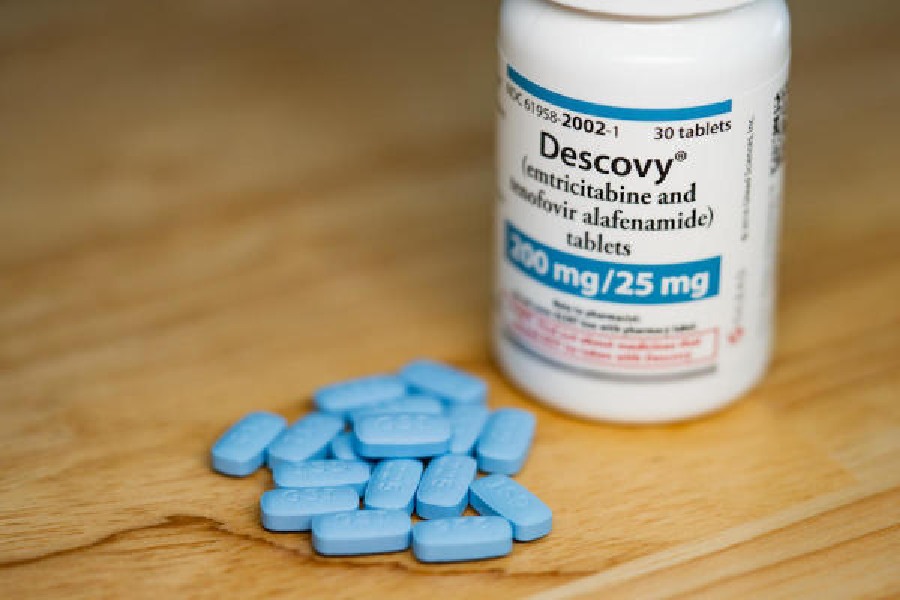
Today, a generation of expensive Gilead drugs containing the new iteration of tenofovir account for half of the market for HIV treatment and prevention, according to IQVIA, an industry data provider. One widely used product, Descovy, has a sticker price of $26,000 annually. Generic versions of its predecessor, Truvada, whose patents have expired, now cost less than $400 a year.
If Gilead had moved ahead with its development of the updated iteration of the drug back in 2004, its patents either would have expired by now or would soon do so.
“We should all take a step back and ask: How did we allow this to happen?” said James Krellenstein, a longtime AIDS activist who has advised lawyers suing Gilead.
Gilead’s manoeuvre with tenofovir is so common in the pharmaceutical industry that it has a name: product hopping. Companies ride out their monopoly on medication and then, shortly before the arrival of generic competition, they switch — or “hop” — patients over to a more recently patented version of the drug to prolong the monopoly.