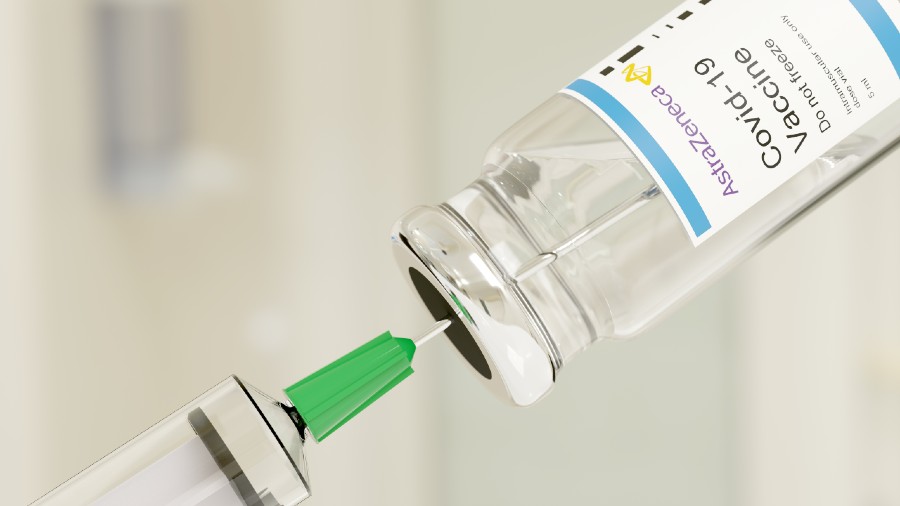
Europe’s medicine regulator on Friday approved the AstraZeneca and Oxford University’s Covid-19 vaccine for people over the age of 18, the third vaccine to be cleared for use in the EU.
The AstraZeneca vaccine demonstrated an efficacy of around 60 per cent in the trials on which the decision was based, the European Medicines Agency (EMA) said in a statement.
That is well below the level of protection shown by authorised vaccines from Pfizer and its German partner BioNTech and rival Moderna, which were around 95 per cent effective in preventing symptomatic illness in pivotal trials.
“With this third positive opinion, we have further expanded the arsenal of vaccines available to EU and EEA member states to combat the pandemic and protect their citizens,” said Emer Cooke, executive director of EMA.
The EU last year agreed to buy up to 400 million doses of the vaccine and has been at the centre of a dispute with AstraZeneca this week over the speed of supplies.
Europe urgently needs more shots to speed up its inoculation programme with Pfizer and Moderna also facing difficulties in delivering the quantities promised for the early months of the year.
The AstraZeneca vaccine is cheaper and easier to store than the two already approved from Pfizer and Moderna.
AstraZeneca CEO Pascal Soriot welcomed the decision.
“Today’s recommendation underscores the value of AstraZeneca’s Covid-19 vaccine which is not only effective and well-tolerated, but also easy to administer and, importantly, protects fully against severe disease and hospitalisations,” he said in a statement.
The AstraZeneca vaccine is administered via two injections into the arm, the second between 4 and 12 weeks after the first. There were not yet enough results for people over the age of 55 to determine how well the vaccine would work for this group, EMA said.