This isn’t the worm’s first brush with international stardom nor is it the first time Caenorhabditis elegans has been thanked for aiding award-winning work. Gary Ruvkun dubbed the Caenorhabditis elegans, which happens to be his experimental subject, “badass”. But Ruvkun’s award was actually the fourth Nobel Prize resulting from C. elegans research, cementing the lowly soil worm’s outsized role in scientific discovery.
The one-millimetre nematode has helped scientists understand how healthy cells are instructed to kill themselves and how the process goes awry in AIDS, strokes and degenerative diseases. (That work was the subject of the 2002 Nobel Prize in physiology or medicine.)
Self-proclaimed “worm people” were recognised by the Nobel committee in 2006 for discovering gene silencing, which became the basis for an entirely new class of drugs. Two years later, the chemistry prize went to scientists who used nematodes to help invent cellular “lanterns” that allowed biologists to see the inner workings of a cell.
For each prize, a laureate made sure to thank the worm, though perhaps the most famous nod came from Sydney Brenner, who won the first “worm Nobel”. “Without doubt, the fourth winner of the Nobel Prize this year is Caenorhabditis elegans,” he said in his lecture in Stockholm.
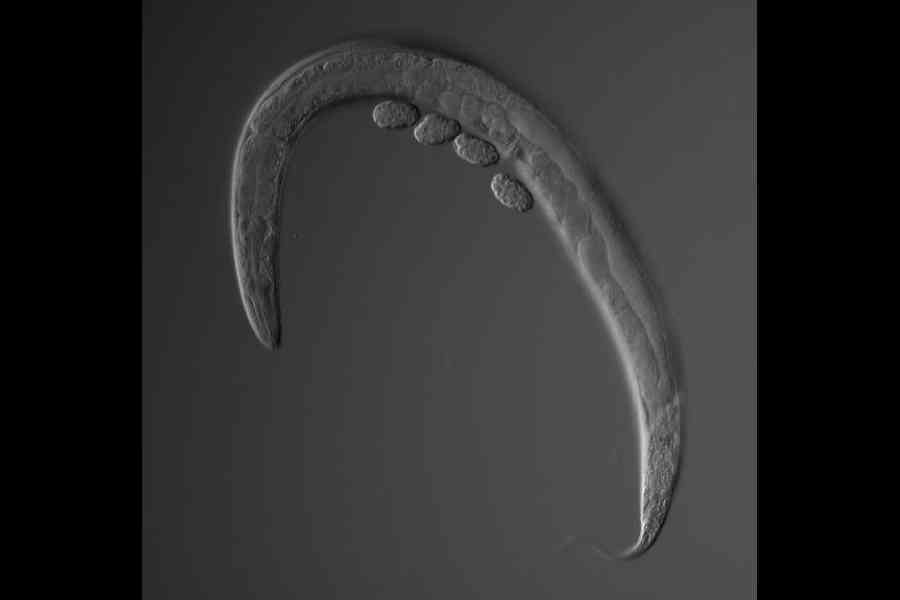
C. elegans is named after the Latin word for “elegant” because of the way it moves in graceful, sinusoidal waves. One of the animal’s virtues is its simplicity, which allows scientists to test hypotheses about fundamental biological concepts in a model that is easy to understand.
The nematodes have just 959 cells — a remarkably manageable number, compared with our trillions of cells — each of which scientists have named and charted from fertilisation to death.
“This is probably the best-understood multicellular organism on the planet,” said Howard Ferris, a nematologist at the University of California, Davis, US.
The destiny of each cell is easy to map, since the worms become translucent under the light of a microscope and cycle through all developmental stages in about three days.
The nematode was the first animal to have its genome entirely deciphered — in 1998, years before scientists were able to do the same for flies and mice. The worm is also inexpensive, easy to store and entirely self-sufficient when it comes to reproduction; female C. elegans have functional sperm that allow them to inseminate themselves.
Even when scientists come to nematology for the worms, they often stay for the tightly knit, offbeat community. Since its inception, the field has had a tradition of collaboration.
J udith Kimble, a nematode researcher at the University of Wisconsin, Madison, US, attributes much of the research success to the fact that worm-bonded scientists tend to share their resources and cooperate, a value she wishes the rest of the country would adopt.
Ruvkun, of the Harvard Medical School, US, and his co-winner, Victor Ambros, a professor of molecular medicine at UMass Chan Medical School, US, shared their findings with each other, allowing them to piece together the mechanics of microRNA. Had they not, their prizewinning work might have been delayed years, maybe even decades.
This collective spirit stands in stark contrast to some other corners of biology, like fly research. But both groups agree that their research is dismissed by mammal scientists, who reside on top of the unspoken lab animal hierarchy and often believe that experiments on invertebrates are irrelevant to humans.
In fact, the discovery of microRNA was first met with silence outside the C. elegans community. It wasn’t until years later, when Ruvkun proved that microRNA was present in a wide array of animals, including humans, that the wider research community finally acquiesced.
NYTNS