Pharmaceutical firm AstraZeneca has accepted an offer from the Russian Direct Investment Fund (RDIF) to use one of the two components of its Sputnik V vaccine in its clinical trials, the institute said on Friday.
On November 20 this year, RDIF and Gamaleya Institute had proposed the pharma giant to use one of the two components (human adenoviral vectors) of the Sputnik V vaccine in the clinical trial of AstraZeneca’s Covid-19 vaccine, Russia's sovereign wealth fund said in a statement.
“AstraZeneca accepted RDIF's proposal and will begin clinical trials of its vaccine in combination with Sputnik V's human adenoviral vector type Ad26 by the end of 2020,” the statement said.
“This research will allow AstraZeneca's scientists to study the possibility of boosting their vaccine's efficacy through the application of this combined approach,” the RDIF said.
“The decision by AstraZeneca to carry out clinical trials using one of two vectors of Sputnik V in order to increase its own vaccine's efficacy is an important step towards uniting efforts in the fight against the pandemic,” RDIF CEO Kirill Dmitriev said.
AstraZeneca had previously come under scrutiny when it accepted a manufacturing error in its vaccine.
“These preliminary data indicate that the vaccine is 70.4% effective, with tests on two different dose regimens showing that the vaccine was 90% effective if administered at a half dose and then at a full dose, or 62% effective if administered in two full doses,” the University of Oxford, with which AstraZeneca had partnered, said in a statement.
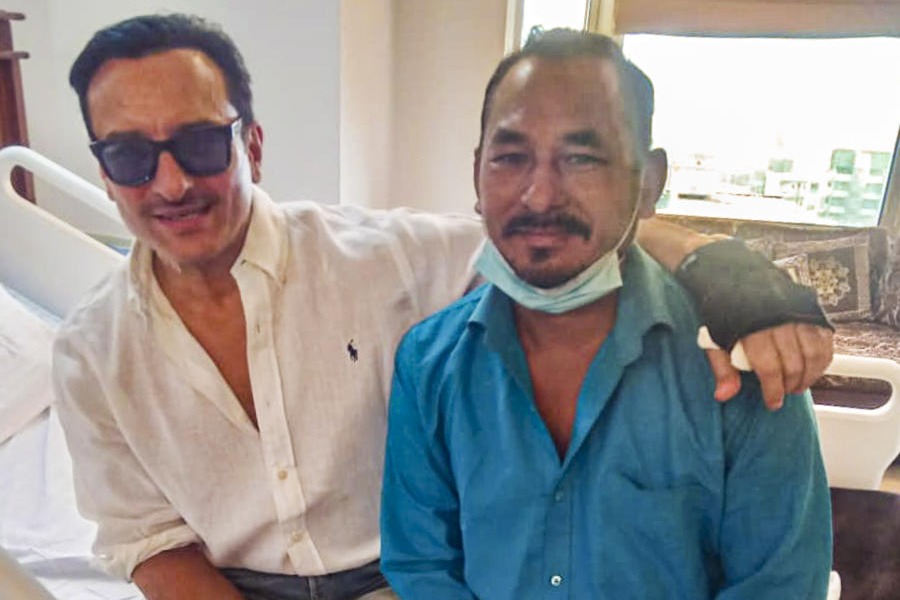
It also faced a slew of questions last month when a Chennai-based volunteer complained of facing neurological and psychological problems after receiving a trial shot of the vaccine.