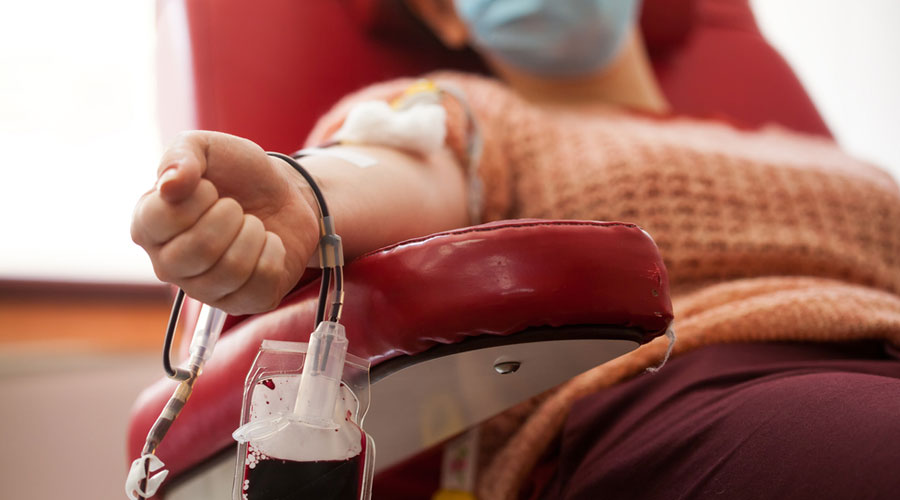
A medical trial in Calcutta and others in India and around the world suggest antibody-rich blood plasma from patients who’ve recovered from Covid-19 can be used to combat the virus and, if administered early, keep people out of intensive care.
The Calcutta study was based on 80 patients at the West Bengal state-run ID&BG Hospital that took place between end-May and end-October. Crucially, it found administering plasma therapy produced “significant immediate mitigation of hypoxia (when oxygen falls to dangerously low levels), reduction in the hospital stay as well as survival benefits” in severely ill COVID-19 patients with Acute Respiratory Disease Syndrome (ARDs).
“It is a therapy that works” in many cases, says Dipyaman Ganguly, principal scientist, Indian Institute of Chemical Biology. Ganguly led the convalescent plasma therapy study which was conducted by the ID&BG Hospital, under a joint initiative of the Centre of Scientific Research (CSIR) and the West Bengal government. The central hope is that antibody therapy could stop mild Covid from turning severe.
While vaccines now are being approved for Covid, it will be months before many people are able to get them and, in the meantime, scientists are hoping to harness the immune system’s natural response to viral invaders.
The study shows “plasma will provide antibodies that will prevent the virus from infecting cells … (and that) other proteins also are circulating which we think are also playing a role in the recovery,” he says. Those patients the therapy benefited were aged below 67 years, said the study.
The Calcutta findings got a shoutout tweet from Dr Arturo Casadevall, chairman of the immunology department at the US Johns Hopkins School of Public Health and who has been a big advocate of the use of convalescent plasma therapy to treat Covid. Casadevall has been running trials that indicate convalescent plasma is most useful in preventing the onset of severe illness or to prevent symptoms completely than as a cure for Covid when it reaches an advanced stage. “Early use is associated with the best outcome,” he says.
Convalescent plasma therapy involves extracting antibodies from the blood of recovered Covid patients and injecting it into coronavirus patients to combat the virus and also into people at risk of contracting the illness. The low-cost therapy itself is a century-old one used before the emergence of antibiotics made convalescent plasma largely obsolete. It won the Nobel Prize in 1901 for curing diphtheria in children.
In recent years, doctors have used plasma to treat Ebola, SARS and MERS when nothing else worked. The beauty of convalescent plasma treatment is that its use “requires no research or development,” as it “relies on standard blood-banking practices,” says Casadavell. While vaccines should confer long-lasting immunity by triggering the recipient to create their own antibodies, receiving antibodies would only get temporary Covid immunity.
Another confirmatory study
Privately run Max Super Speciality Hospital also has just published a study on patients in the intensive care unit that were administered plasma therapy and which showed “mortality was significantly lower in the plasma group.” That study focused on late-stage Covid patients -- some 694 patients who were admitted to the intensive care unit. Of these, 333 were given plasma with the best supportive care and the remaining 361 received the best supportive care only. That study found that the mortality rate in Covid patients administered plasma was 25.5 per cent against 33.2 per cent among those who did not receive the plasma. Unlike some other studies, this found good outcomes in relatively late usage of plasma therapy.
This benefit of reduced mortality was most seen in those aged 60 to 74 years -- 26.7 per cent versus 43.0 per cent. Women in this age group seemed to benefit especially from plasma treatment. There was also a “significant difference” in mortality observed in patients with one comorbidity (22.3 per cent against 36.5 per cent).
Moreover, patients on ventilators had significantly lower mortality rates in the plasma group, 37.2 per cent against 49.3 per cent in the non-plasma group. The plasma effect was particularly marked for patients who were on mechanical ventilation. The death rate in the plasma group was 63.9 per cent against 82.9 per cent for those in the non-plasma group.
The ICMR study, its drawbacks
These findings conflict with the conclusions of an ICMR double-blind trial that involved 433 participants. The study covered 39 hospitals across India. ICMR reported convalescent plasma was “not associated with a reduction in progress to severe Covid-19 or all-cause mortality… we did not find any benefit from convalescent plasma being administered within three days of symptom onset in Covid-19.”
In its study, the ICMR noted some possible drawbacks including the fact that most of the donors only had mild Covid and that it could not measure the antibody levels in the convalescent plasma before giving it to patients because reliable tests for antibody measurement weren’t available when the trial began.
The ICMR researchers noted that those people who had severe Covid were reluctant to return to the hospital to donate plasma. “This has major implications for obvious operational reasons in the scaling up of convalescent plasma treatment for Covid-19 not only in India but also globally,” the ICMR said.
A US study suggests that 30 per cent of documented Covid patients are not making enough antibodies to qualify for plasma donation as they have mild cases. Ultimately, some researchers say while plasma might be useful right now, in the long-run, it might be more advantageous to identify the best antibodies to Covid, then synthesise them in labs. These are known as "monoclonal antibodies" and they are being developed by biotech firms including Regeneron and Eli Lilly. US President Trump received a drug produced by Regeneron.
Levels of antibodies critical
High levels of antibodies in the donated plasma are seen by Casadevall, the Johns Hopkins immunologist, and other researchers as being critical to plasma therapy being effective. High antibody levels occur in people who have had a fairly severe bout of Covid-19.
Secondly, in patients treated early, mortality rates are lowest when the plasma contains high antibody levels. The mortality rates are highest in patients who received low antibody plasma, and somewhere around the middle when levels of antibody are in the intermediate range.
Recovered patients can become donors after three-to-four weeks of testing negative. The ICMR said in a later statement later that a potential donor for convalescent plasma should have sufficient concentration of neutralising antibodies before giving it to patients
In Kuwait, meanwhile, an observational study found significant reductions in mortality with high antibody convalescent plasma if it is given within 24 hours of hospital admission. Another study carried out in the Bronx in New York City found that plasma recipients suffering from severe Covid-19 had a four-fold lower mortality rate at day 28 of the disease.
Funded by Bill and Melinda Gates Foundation
A further double-blind study in Argentina funded by The Bill and Melinda Gates Foundation of 160 elderly subjects over the age of 64 found that administration of high-antibody convalescent plasma against Covid to mildly ill infected seniors within 72 hours of mild Covid symptoms reduced the virus’s progression.
Meanwhile, early findings of a US study, which enrolled more than 35,000 patients, found that quickly administering so-called convalescent plasma had a marked effect on mortality for patients with severe cases of Covid-19. Those who received transfusions within three days of diagnosis had a seven-day death rate of 8.7 per cent, while patients who got plasma after four or more days had a mortality rate of 11.9 per cent in the study, run by the Mayo Clinic.
The study enrolled a high proportion of critically ill patients, with about 52 per cent in intensive care units and 28 per cent requiring mechanical help to breathe. In this study, researchers also found that the quality of the plasma infusion had an effect on patient outcomes, as those who got infusions that were particularly rich with antibodies fared better overall.
While doctors like best to rely on randomised control studies, observational trials also can be useful. The efficacy of penicillin is an example of a drug accepted without randomised trials.
“In this kind of treatment, there will be some who respond and some who don’t,” says Ganguly. “There’s a need to categorise them and how to recognise parameters of a responder,” he says.