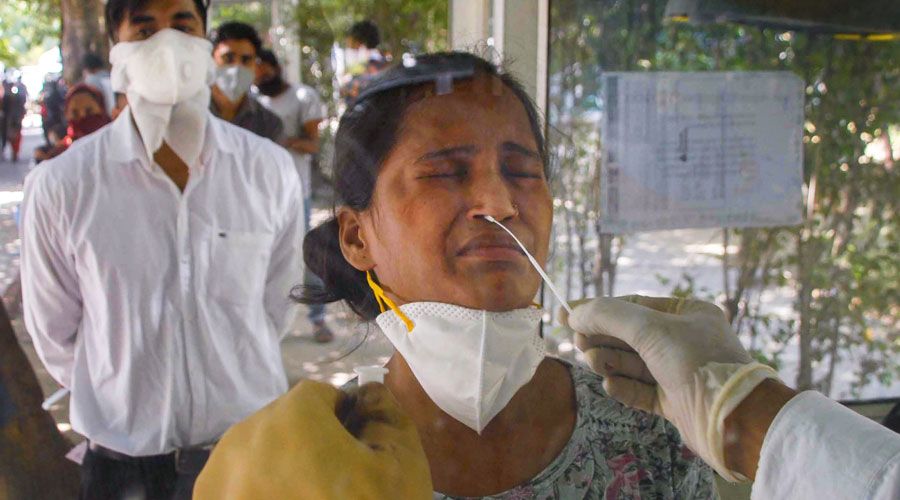
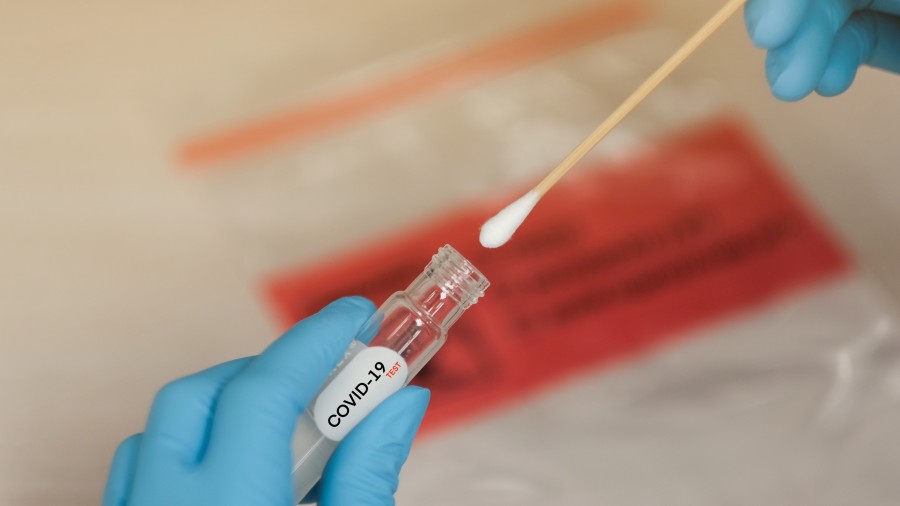
India’s health research agency on Thursday signalled its intention to use antibodies extracted from horse blood to treat patients with severe coronavirus disease, planning to extend a decades-old strategy used against tetanus, rabies and snakebites.
The Indian Council of Medical Research (ICMR) and the Hyderabad-based company Biological E have developed “highly purified antisera” — serums loaded with antibodies against SARS-CoV-2, the virus that causes Covid-19 — for possible use on patients, the ICMR announced.
The equine — or horse-derived — antisera are expected to serve as a fast-acting therapy for patients with severe Covid-19 but would need to be evaluated through human clinical trials, a senior ICMR official told The Telegraph.
Horses have to be exposed to the infection for them to develop antibodies in their bloodstream.
Researchers in Argentina and Brazil are also pursuing equine antisera. Studies in Brazil have suggested that equine-produced serum is superior to the convalescent plasma extracted from patients who have recovered from Covid-19.
“We expect equine antisera to be a standardised product with antibody concentrations much higher than those present in convalescent plasma,” the ICMR official said. While antisera and convalescent plasma both contain virus-neutralising antibodies, researchers expect the antisera to be more effective because of higher antibody concentrations.
A nationwide trial on convalescent plasma conducted by the ICMR earlier this year had not found any effect on either mortality or severe coronavirus disease. Some scientists have speculated that the lack of effect could have been a result of low antibody concentrations in the plasma.
The ICMR official said the clinical trials on the antisera were likely to be conducted only on patients with moderate to severe disease but did not specify any timetable for the trials.
“There is no vaccine against Covid-19 on the horizon yet — the antisera strategy has been used against diphtheria, tetanus, rabies and snakebites. This could be tried against Covid,” asenior clinician-researcher said.
The antisera therapy is designed to work like a medicine. While the body’s immune system begins to mount an attack shortly after the viral infection, researchers say, if the viral load is particularly large, there is a window of time before the immune system can clear the virus.
The antisera represents a potential complementary strategy to treat Covid-19 which currently relies primarily on supportive therapy, steroids, oxygen and experimental medications such as the anti-virals remdesivir and favipiravir and anti-inflammatory drugs.
Brazilian researchers had in August published a report showing equine antisera as superior to convalescent plasma. They had estimated that because one horse can be bled around six times a year without causing the animal any suffering, they could produce 100,000 ampoules, or doses, per year relying on 80 horses.
While equine antisera containing a mix of antibodies has been in use as therapy in other infections, some scientists have underlined that research groups across the world are trying to develop “monoclonal antibodies” — a superior alternative to the equine antisera that may be associated with side-effects.