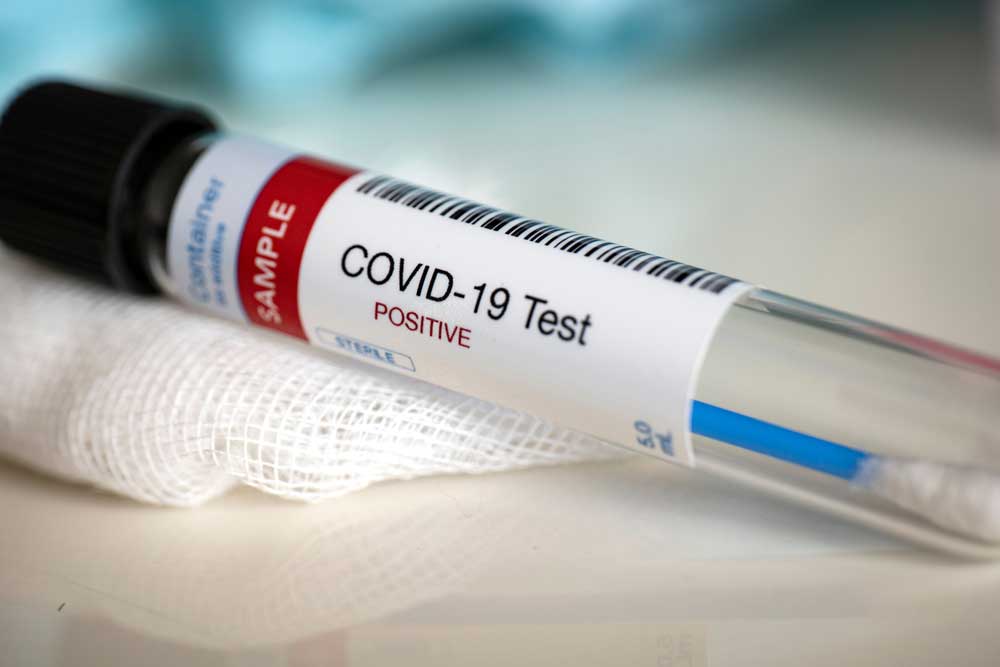
India’s health research agency announced on Sunday it had developed and validated a rapid antibody test to detect the new coronavirus infection, rekindling hopes of large-scale surveillance after a setback two weeks ago.
On April 27, the Indian Council of Medical Research had rejected similar test kits procured from China, citing field failures.
Now the council’s own scientists at the National Institute of Virology, Pune, have developed an indigenous Covid-Elisa test that will detect antibodies in blood samples to reveal whether a person has been infected, the ICMR said.
It said it had already validated the test and entered into a partnership with the Ahmedabad-based pharmaceutical company Zydus Cadila for mass production of the kits.
The test has much higher sensitivity and specificity than the imported kits, the ICMR said without quantifying the differences.
Poor sensitivity and specificity values can lead, respectively, to false negatives (an infected person being shown as uninfected) and false positives (an uninfected person being shown as infected).
The ICMR said the indigenous Covid-Elisa test had been validated at two sites in Mumbai and could screen 90 samples together in a single run, yielding results within two-and-a-half hours.
While the diagnostic test for Covid-19 looks for viral genetic material in patients’ throat or nasal swabs, the antibody tests look for anti-viral antibodies generated by people who are or have been recently infected.
“The antibody tests are critical for mass surveillance across large populations,” a senior medical researcher said.
Such surveillance programmes can provide insights into what proportions of people in specific localities or even districts have been infected.
The worldwide infection patterns suggest that the coronavirus causes only mild symptoms in around 80 per cent of the infected people. Clinical virologists say this raises the possibility that some of those infected are not aware of it because they did not seek the diagnostic test.
Health authorities recorded 3,277 new Covid-19 cases on Sunday, raising India’s number of patients to 62,939, including 2,109 deaths. Some 19,358 patients have recovered and 41,472 are still in hospital.
But health experts say the country’s true burden of the infection remains unknown as infected people without symptoms have not been counted, nor have those with only mild symptoms or those who have not been tested.
The antibody tests can help determine current or recent infection in people who may not have the symptoms any more, a senior virologist said.
A medical panel set up by the ICMR had last month proposed surveillance for the coronavirus through antibody tests on samples of households in all the districts.
After the ICMR rejected the Chinese kits, the panel had proposed an alternative, scaled-down surveillance programme, through the standard viral diagnostic test, to screen patients and healthcare workers in 80 district hospitals.