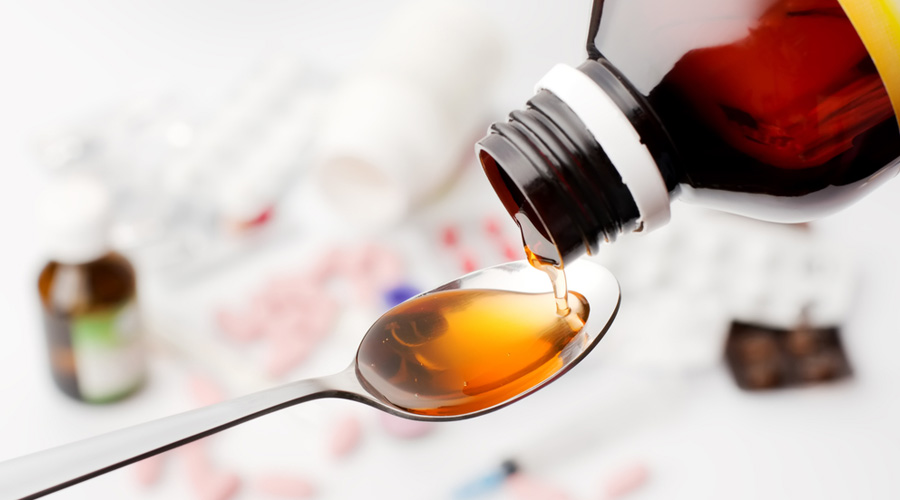
The World Health Organisation and a Gambian parliamentary panel have reaffirmed that the cough syrups imported from India and linked to deaths of Gambian children were contaminated with toxic chemicals, days after India’s health ministry said samples from the exporter had been found clean.
Laboratories in Ghana and Switzerland contracted by the WHO have tested the syrups, made by the Haryana-based Maiden Pharmaceuticals, and confirmed unacceptably high levels of diethylene glycol (DEG) and ethylene glycol (EG) in them, the WHO and the panel have said. The two chemicals can cause acute kidney injury and lead to death.
These contaminants “are dangerous and should not be in any medicine ever”, a WHO spokesperson told The Telegraph.
The spokesperson was responding to a query seeking the WHO’s perspective on assertions by India’s apex drug regulator that neither the WHO nor the Gambian authorities had provided evidence that the syrups actually caused the deaths.
The observations by the WHO and the Gambian panel come amid what some experts believe are efforts by the Indian government to shift the focus from contamination to the absence of evidence to establish the exact cause of the deaths.
The Gambian National Assembly panel that investigated the matter said it “is convinced that Maiden Pharmaceuticals is culpable and should be held accountable for exporting contaminated medicines” linked to the deaths of at least 66 children in The Gambia earlier this year.
Drugs Controller General of India (DGCI) V.G. Somani had earlier this month complained to the WHO that the global health agency had not, despite multiple requests from India, provided any details to establish causality between the syrups and the deaths.
Somani had in his letter to the WHO said that tests at a government lab on samples of syrups collected from Maiden had failed to detect the toxic chemicals. He had asked whether the WHO had made a “premature deduction” on the cause of the deaths.
But experts say that a cause-effect link is difficult to establish without sophisticated tests on tissue samples.
A member of an expert panel set up by the Central Drugs Standard Control Organisation (CDSCO), the country’s top drug regulatory authority and an arm of the health ministry, told this newspaper that the panel had not received any evidence of a cause-effect link between the syrups and the deaths.
But experts in pharmacology and forensic medicine said that the emergence of a sudden cluster of several children with symptoms of acute kidney injury after they had consumed the cough syrups would itself represent epidemiological evidence to suspect the syrups.
“The cluster of cases provides the circumstantial evidence,” said B. Suresh Shetty, professor of forensic medicine at the Kasturba Medical College, Mangalore. “It allows health authorities to ask questions — for example, did the cases have anything in common.”
The Gambian parliamentary panel, in its report released on December 20, said that 21 children brought to the emergency paediatric unit with inability to pass urine had received the syrups one to six days earlier. Most of the children had developed the symptom two to three days after taking the drugs, the panel said.
“In such circumstances, it is very difficult to prove cause-effect — that would require at the very least biochemical and toxicity analysis of the kidney tissues and sophisticated tests,” said Bhavneet Bharti, earlier professor of paediatrics at the Postgraduate Institute of Medical Education and Research, Chandigarh, and now at the BR Ambedkar State Institute of Medical Sciences, Mohali.
The WHO’s global alert on October 5 about four syrups produced by Maiden had been preceded by weeks of investigations by the Gambian Medicines Control Agency and the WHO’s Gambia office.
The Medicines Control Agency had issued alerts on September 10 and 16, flagging “suspect cases of reported deaths of children” after the consumption of syrups. On September 21, the agency said “all brands containing paracetamol and promethazine syrup(s)” had been included as “suspected product(s)” in the cases of acute kidney injury and deaths in children.
The Gambian parliamentary panel has concluded that all the acute kidney injury cases were linked to the consumption of the contaminated syrups from Maiden, but added that “the actual cause of death of these children is still under investigation”.
The CDSCO’s expert panel member told this newspaper that the CDSCO had sought but not received any data from the WHO or from the Gambian authorities on how many of the 66 children who died had consumed the Maiden syrups.
The WHO spokesperson said that when many children die of a mysterious illness, “it’s a tragedy that means WHO had to act quickly”.
“WHO’s mandate is to issue global alerts about potential risks. WHO stands by the action taken,” the spokesperson said.