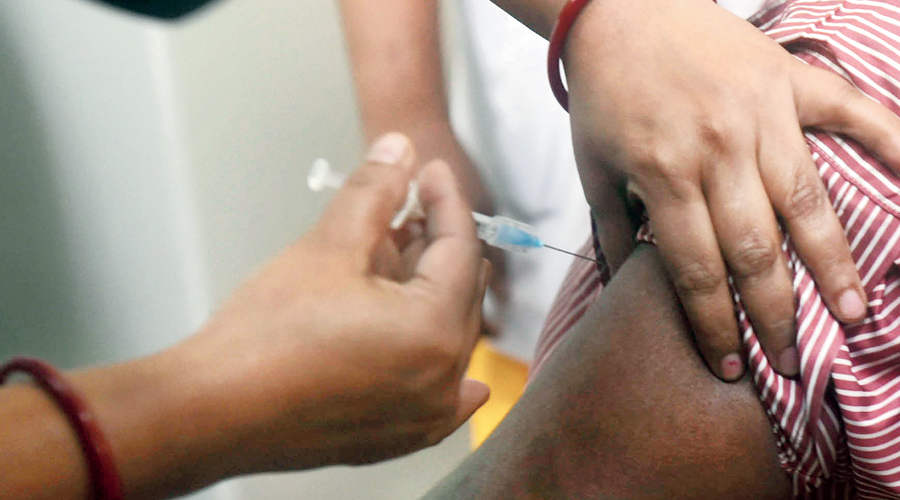
India has administered around 45 million fewer Covid-19 vaccine doses during May than it had during April despite the campaign opening this month for all adults 18 years or older and expanding the number of beneficiaries.
The Union health ministry’s daily vaccination reports show that India had administered only around 44 million doses between May 1 and May 25 compared with over 89 million doses during April. The total number of doses given was 200,662,456, or over 20 crore, until 7am on Wednesday.
The vaccination campaign has over the past week administered an average of about 1.5 million doses every day, compared with an average 2.7 million daily doses during the corresponding week in April. Even if the campaign marks 3 million doses every day for the rest of the month, the cumulative doses in May will be less than 63 million, or about 70 per cent of the vaccines administered in April.
Health experts tracking the vaccination campaign are puzzled by the decline in the doses administered at a time when people aged 18 years or older are scrambling for vaccines in states across the country.
“Something must be wrong somewhere — either we’re not being told about hiccups that might have interrupted smooth production or the government has overstated the production figures,” said R. Ramakumar, an economist and professor of development studies at the Tata Institute of Social Sciences, Mumbai.
The government’s vaccination campaign currently relies on Covishield, the AstraZeneca vaccine produced by the Serum Institute of India, and Covaxin, the homegrown vaccine from Bharat Biotech.
The Centre, in an affidavit submitted to the Supreme Court on May 9, had said the Serum Institute had ramped up production to 65 million doses per month and Bharat Biotech had increased manufacturing to 20 million doses per month.
Ramakumar and others believe a production capacity of 85 million doses per month, or about 2.8 million doses every day, should ideally translate into at least 2 million or 2.3 million doses administered daily.
Neither the health ministry, nor the Serum Institute, nor Bharat Biotech responded to queries sent by The Telegraph asking about the fewer doses administered during May compared with the doses in April.
A senior health ministry official had said earlier this week that rollouts take place only after vaccines pass through stability, sterility and quality checks and this process can take an extra seven to 10 days after production.
But vaccine experts familiar with production processes say such post-production checks are routine and occur with every batch of vaccines.
“If batch-to-batch production is smooth and uninterrupted, the 7-to-10-day lag should not impact rollout at all,” one expert said. “Each subsequent batch makes up for the lag and procurements and rollout should be smooth.”
The Centre had on April 19 announced a revised vaccination strategy under which it would procure 50 per cent of available vaccine doses that would go into inoculating people 45 years or older and states and private hospitals could procure the balance 50 per cent to vaccinate people 18 years or older.
Sections of public health experts have described the Centre’s decision to expand the vaccination campaign without making provisions for extra vaccines as an attempt to make states share the blame for vaccine shortages that were imminent.