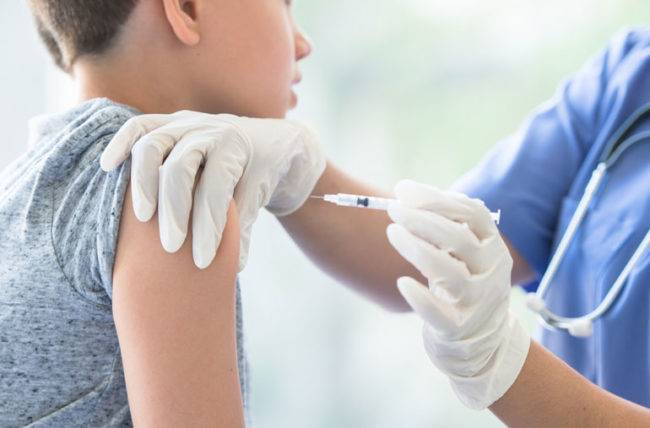
An expert panel of India’s central drug authority has recommended granting emergency use authorisation (EUA) for Biological E’s Covid-19 vaccine Corbevax for children aged between five and 11 years, official sources said on Thursday.
The Subject Expert Committee on Covid-19 of the CDSCO has sought more data from Bharat Biotech to review its EUA application for the use of Covaxin in the 2-to-11 age group, they said.
The Drugs Controller General of India had on December 28 approved Covovax by Novavax for restricted use in emergency situations in adults and in the 12-to-17 age group subject to certain conditions on March 9 this year.
Biological E’s Corbevax is being used to inoculate children aged between 12 and 14.
Covaxin has been granted Emergency Use Listing (EUL) by the DCGI for the age group of 12 to 18 years on December 24, 2021.
“The Subject Expert Committee on Covud-19 of the CDSCO which deliberated on Biological E's EUA application has recommended granting emergency use authorisation for use of Corbevax in the age group of five to 11 years,” a source said.
India began inoculating children aged 12-14 on March 16.
The countrywide vaccination drive was rolled out on January 16 last year with healthcare workers getting inoculated in the first phase.
Vaccination of frontline workers started from February 2 last year.
The next phase of Covid-19 vaccination commenced on March 1 last year for people over 60 years of age and those aged 45 and above with specified co-morbid conditions.
India launched vaccination for all people aged more than 45 years from April 1 last year. The government then decided to expand its vaccination drive by allowing everyone above 18 years of age to be inoculated against the viral disease from May 1 last year.
The next phase of vaccination commenced on January 3 for adolescents in the age group of 15-18 years.
India began administering precaution doses of vaccines to healthcare and frontline workers and those aged 60 and above with comorbidities from January 10.
Precaution does of Covid-19 vaccines to all aged above 18 years was allowed at private vaccination centres from April 10.
TN surrogacy leave
Chennai: The Tamil Nadu government will grant 270 days’ leave to women employees and teachers to care for their children got through surrogacy. PTI