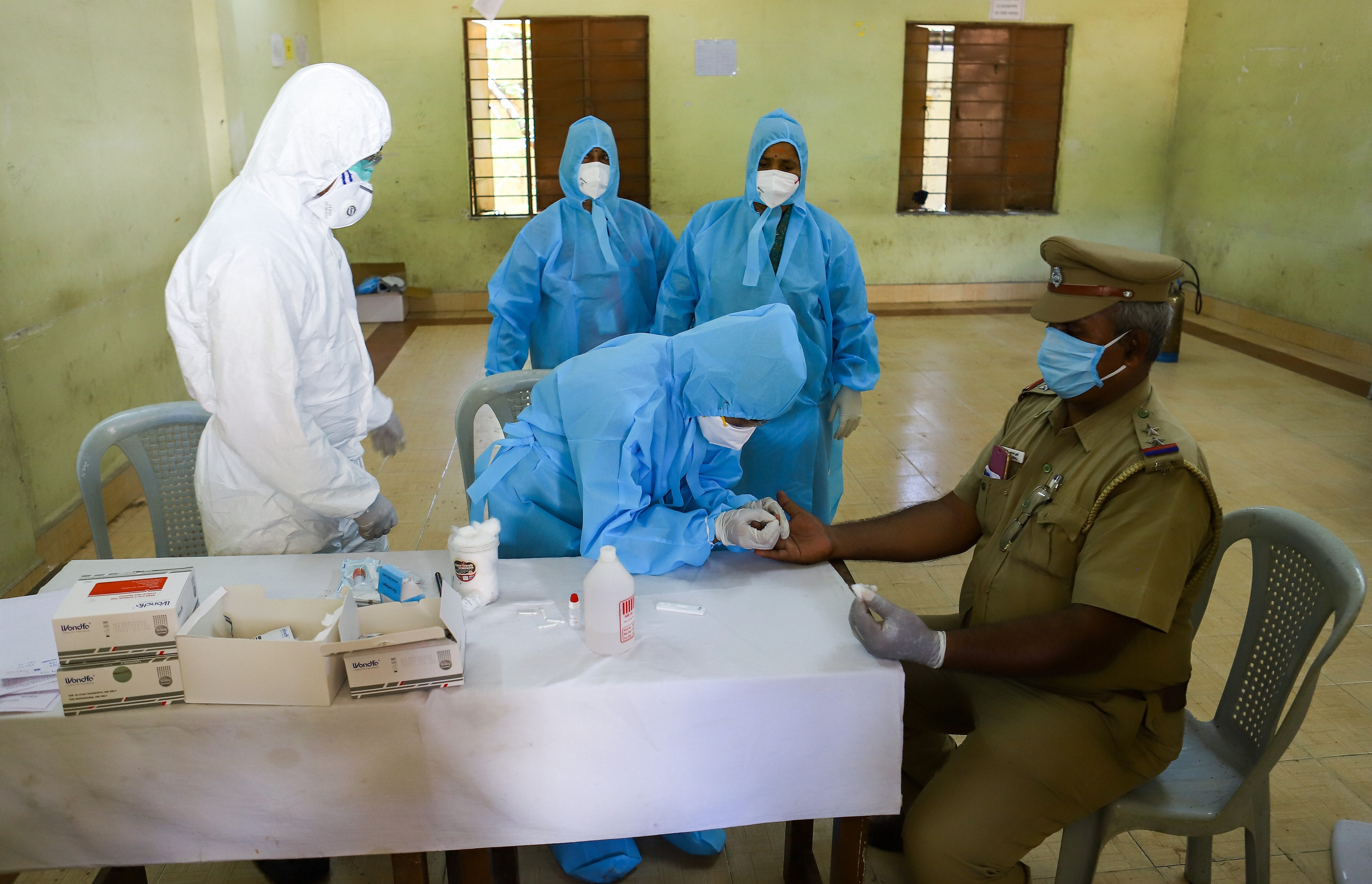
States and Union Territories have been asked to put on hold the use of Covid-19 rapid antibody test kits till their accuracy is rechecked by apex health research body the Indian Council of Medical Research (ICMR), official sources said on Saturday.
According to the sources, teams constituted by ICMR are analysing the rapid antibody test kits, procured from two Chinese firms, to check their efficacy after some states reported that they are faulty and giving inaccurate results.
Union Health Minister Harsh Vardhan on Friday said the results of the test kits vary from place to place and 'it can't be relied upon'.
'Moreover, WHO has also not commented on its accuracy. ICMR is reviewing the efficacy of the test and the kits in its own labs and shall come out with fresh guidelines soon,' he was quoted as saying in a health ministry statement on Friday.
An official said, 'After a meeting the Union health ministry held with states on Friday, they were asked to halt the use of COVID-19 rapid antibody test kits for the time being till their accuracy is validated by the ICMR.'
Head of Epidemiology and Communicable Diseases at ICMR, Dr Raman R Gangakhedkar at a press briefing on Tuesday said high variations between the results of the rapid tests and RT-PCR tests, ranging from 6 to 71 per cent, were reported in some states. He had also said that if batches of the rapid test kits are found to be faulty, the company will be asked to replace them.
India has recently procured five lakh rapid antibody test kits from two Chinese firms and they were distributed to states for districts with high burden of Covid-19 infection.
The ICMR on April 22 had issued a protocol reiterating the usage of these kit for surveillance purpose while stating that RT-PCR tests must be continued vigorously as the principal diagnostic test for Covid-19.
'The ICMR has always emphasised that the confirmatory test for diagnosis of Covid-19 infection is RT-PCR test of throat and/or nasal swab for detection of the virus at early stage,' the apex health research body had said.
At present, the government uses the polymerase chain reaction (PCR) test from throat or nasal swab samples of people to detect coronavirus. This test takes around five to six hours to show results. In the rapid antibody test, blood samples of suspected cases are collected for examination and the wait period for the result is around 15-30 minutes.
Earlier the government had said that rapid antibody test kits are to be used for surveillance and epidemiological purposes in coronavirus hotspot areas.
'The RT-PCR test is the gold standard for frontline test and antibody test cannot replace this. Utility of rapid antibody test is primarily for assessing prevalence of infection in a particular area,' an official had said earlier.