The US Food and Drug Administration (USFDA) has issued an import alert on the Halol unit of Sun Pharmaceutical Industries.
All future shipments of products manufactured at the facility are subject to the refusal of admission to the US market until the facility becomes compliant with cGMP (current good manufacturing practice) standards.
It follows an inspection of the facility by the US drug regulator from April 26 to May 9, 2022.
“We now wish to inform you that the company has received a communication from the USFDA stating that the facility has been listed under import alert,” Sun Pharma said in a regulatory filing.
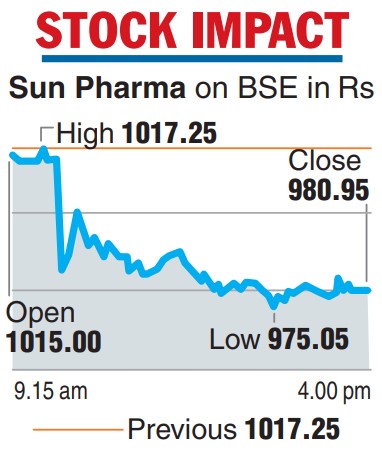
The disclosure led to the shares of the country’s top drug maker falling 3.57 per cent or Rs 36.30 on the BSE to Rs 980.95. Sun Pharma was the top loser in the Sensex pack on a day the benchmark index rose 160 points.
Sun Pharma added that the USFDA has excluded 14 products from this import alert, subject to certain conditions.