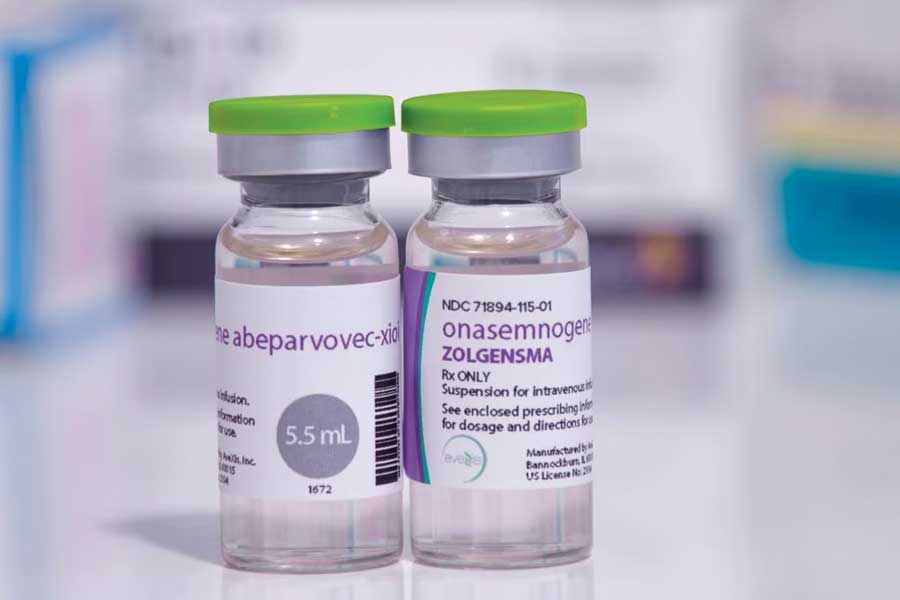
A staggering Rs 17 crore for a single jab! That’s the cost of one Zolgensma injection used for children under two suffering from spinal muscular atrophy (SMA), the disease and its treatment once again in the headlines over a 15-month-old boy in Karnataka staring at an uncertain tomorrow.
The gene therapy, one of the world’s most expensive drugs, offers promising results but experts caution that it is not a definitive solution for the rare nerve condition that leads to muscle wasting and weakness. It can’t cure but limits the progress of the disease.
Karnataka Chief Minister Siddaramaiah recently wrote to Prime Minister Narendra Modi seeking help for the treatment of a 15-month-old child from the state diagnosed with SMA.
Though it is yet to be approved in India, insiders estimate that about 90 children have received Zolgensma in the last few years. Given that the single shot infusion is out of reach for everybody except the super-rich, most of this is through humanitarian programmes or crowd funding. The drug can be imported following a doctor’s recommendation and government approval.
Worldwide, the incidence of SMA is one in 10,000 live-born babies and is the leading cause of death in the infantile age group.
“In the West, the carrier frequency - number of people carrying the gene - of the SMA is 1 in 50. In a recent Indian study, however, the SMA carrier frequency was identified as 1 in 38. Another study from north India found it to be 1 in 30,” said Sheffali Gulati, child neurologist, All India Institute of Medical Sciences (AIIMS), New Delhi.
“The survival of children with SMA type 1 has increased recently with the availability of supportive care such as physiotherapy, nasogastric feeding (through a tube) and invasive or non-invasive respiratory care,” Gulati told PTI.
As concern mounts over the incidence of the disease and the astronomical cost of the single injection, experts decode what it exactly is: WHAT IS ZOLGENSMA? Zolgensma, developed by Swiss pharmaceutical company Novartis, is designed to treat SMA, a rare genetic disease affecting motor neurons - complex circuits throughout the body that allow for movements of glands and muscles. SMA is caused by mutations in the SMN gene, leading to muscle weakness and, in severe cases, paralysis and even death.
“Zolgensma delivers a functional copy of the SMN gene into motor neuron cells, improving muscle movement and function in children with SMA,” explained Dr N Varsha Monica Reddy, consultant paediatric neurologist, Yashoda Hospitals, Hyderabad.
“The gene encodes the SMN protein - found throughout the body, which is critical for the maintenance and function of motor neurons. In the absence of enough functional SMN protein, motor neurons die, leading to debilitating and often fatal muscle weakness,” Reddy told PTI.
Why is it so expensive?
Paediatrician Vibhu Kawatra noted that Zolgensma’s cost at approximately Rs 17 crore (USD 2.1 million) in India (minus taxes) is due to the extensive research and development involved.
“The limited market size and its potential to save lives contribute to the high price. Despite its initial expense, experts argue that Zolgensma’s long-term benefits may offset healthcare costs associated with SMA treatment and care,” Kawatra, a visiting consultant at Delhi’s Rainbow Children's Hospital, told PTI.
“Zolgensma is the most effective drug for the disorder. The reason for its exorbitant cost is its minuscule market size in the drug manufacturing industry and its potential to save lives,” Reddy added.
Who can use it?
Zolgensma is specifically designed for children under two who are diagnosed with SMA through genetic testing. It is a one-time infusion administered over one hour, aiming to improve muscle movement and developmental milestones in SMA patients.
“To my knowledge, roughly over 90 patients have been treated by intravenous Zolgensma all over the country. Of these, 75 to 80 per cent have received dosing via the humanitarian access programme and the rest through crowd funding or support from employee beneficiary scheme,” said Gulati.
“We have so far administered intravenous doses for about 18 patients in our centre at AIIMS. Fifteen were through humanitarian access programmes, one patient through crowd funding, and two patients through employee beneficiary scheme.” Novartis has provided this therapy free of charge to nearly 300 children in 36 countries, the company said. It has done this through its global Managed Access Program (gMAP), which is available in countries where Zolgensma has not yet received approval.
How effective is it?
The US Food and Drug Administration (FDA) on May 24, 2019, approved onasemnogene abeparvovec for the treatment of SMA. The drug has been marketed under the trade name Zolgensma.
Kawatra believes the therapy is not an endpoint solution for the disease, but it does stall the progress of the disease to a large extent.
“You only have up to two years when you can use this drug. So, for sure, there's not a complete solution. It's just that this drug is something that curtails and restricts the spinal muscular atrophy which is happening. It just kind of limits,” he explained.
Status in India
As of now, Zolgensma is neither approved nor manufactured in India, said Reddy.
“Patients diagnosed with SMA can import the drug from the US, following a doctor's recommendation and government approvals. In India, the approved SMA treatment option is Evrysdi, manufactured by Swiss multinational healthcare company Roche,” she said.
Side effects
The most common side effects are elevated liver enzymes and vomiting. A patient’S liver function should be monitored for at least three months after Zolgensma administration, said Reddy.
Other available treatments for SMA
Apart from Zolgensma, there are other treatments available for SMA patients. Spinraza (nuninersen), approved by the US FDA in December 2016, is an injection administered into the spinal cord fluid. Additionally, Evrysdi (risdiplam), the first oral drug for SMA, was released in August 2020, catering to patients as young as two months.
A study concluded that Zolgensma is more cost-efficient than alternatives such as Spinraza, according to an article published in the journal Cureus in March this year.
“Evrysdi is a daily oral medication that must be taken for the duration of the individual’s life. Spinraza is administered via an intrathecal injection with four loading doses in the first two months of treatment, followed by a maintenance dose every four months for the duration of the individual’s life,” the authors said.
Intrathecal involves injecting the drug into the plasma contained in the brain, cranium and spine.
“The cost of Spinraza is approximately Rs 4.2 crore (INR) for the first year and thereafter Rs 2.1 crore per year lifelong. Risdaplam (Evrysdi) is an oral medication for adults and children older than two months of age. It has to be taken life long, orally, on a daily basis. Currently, this is the only DCGI (Drugs Controller General of India) approved and marketed drug in India. The approximate cost for a 20 kg child will be around Rs 72.8 lakh per year,” said Gulati.
Except for the headline, this story has not been edited by The Telegraph Online staff and has been published from a syndicated feed.